Chlorine More Electronegative Than Iodine . → atomic radius decreases → ionization energy increases →. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. 119 rows values for electronegativity run from 0 to 4. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. 103 rows periodic table of electronegativity by pauling scale. Electronegativity is used to predict whether. Perbromic acid (hbro 4 ). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear.
from www.gbu-presnenskij.ru
this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. → atomic radius decreases → ionization energy increases →. Perbromic acid (hbro 4 ). 119 rows values for electronegativity run from 0 to 4. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. 103 rows periodic table of electronegativity by pauling scale. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. Electronegativity is used to predict whether.
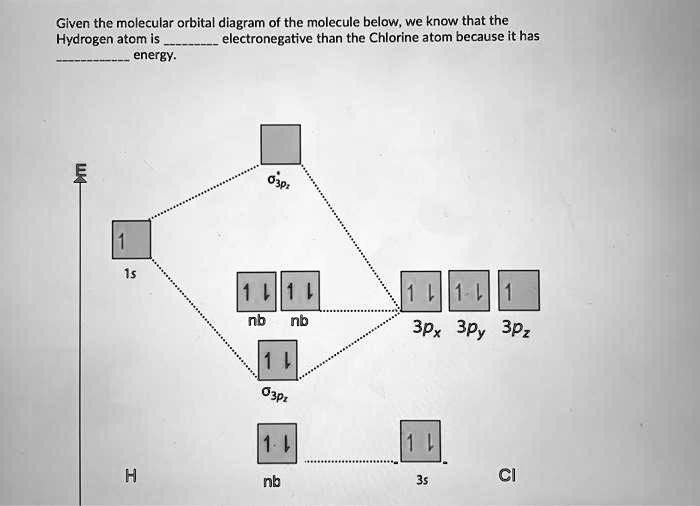
SOLVED Given The Molecular Orbital Diagram Of The Molecule, 45 OFF
Chlorine More Electronegative Than Iodine the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. Perbromic acid (hbro 4 ). → atomic radius decreases → ionization energy increases →. 103 rows periodic table of electronegativity by pauling scale. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. Electronegativity is used to predict whether. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. 119 rows values for electronegativity run from 0 to 4.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Chlorine More Electronegative Than Iodine Perbromic acid (hbro 4 ). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. 119 rows values for electronegativity run from 0 to 4. 103 rows periodic table of electronegativity by pauling scale. atomic chlorine is much more electronegative than atomic bromine, which is more. Chlorine More Electronegative Than Iodine.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Chlorine More Electronegative Than Iodine the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. Perbromic acid (hbro 4 ). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. Electronegativity. Chlorine More Electronegative Than Iodine.
From fr.dreamstime.com
Table Périodique D'Electronegativity Image stock Image 38153931 Chlorine More Electronegative Than Iodine → atomic radius decreases → ionization energy increases →. 119 rows values for electronegativity run from 0 to 4. Electronegativity is used to predict whether. Perbromic acid (hbro 4 ). this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. 103 rows periodic table of. Chlorine More Electronegative Than Iodine.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy Chlorine More Electronegative Than Iodine Electronegativity is used to predict whether. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. → atomic radius decreases → ionization energy increases →. 103 rows periodic table of electronegativity by pauling scale. Perbromic acid (hbro 4 ). chlorine is more reactive than iodine. Chlorine More Electronegative Than Iodine.
From www.numerade.com
SOLVED What will be the product of the addition of ICl to 1 butene Chlorine More Electronegative Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. → atomic radius decreases → ionization energy increases →. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative. Chlorine More Electronegative Than Iodine.
From slideplayer.com
Elemental Properties and Patterns ppt download Chlorine More Electronegative Than Iodine 103 rows periodic table of electronegativity by pauling scale. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. → atomic radius decreases → ionization energy increases →. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. Perbromic acid (hbro 4 ). this article. Chlorine More Electronegative Than Iodine.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Chlorine More Electronegative Than Iodine Electronegativity is used to predict whether. → atomic radius decreases → ionization energy increases →. Perbromic acid (hbro 4 ). 119 rows values for electronegativity run from 0 to 4. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. chlorine is more reactive than iodine due to its higher electronegativity, resulting. Chlorine More Electronegative Than Iodine.
From slideplayer.com
Chemical Bonding II Molecular Geometry ppt download Chlorine More Electronegative Than Iodine this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. Perbromic acid (hbro 4 ). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. → atomic radius decreases → ionization energy increases →. 119. Chlorine More Electronegative Than Iodine.
From exoyzbldb.blob.core.windows.net
Is Chlorine Electronegative Or Electropositive at Otis King blog Chlorine More Electronegative Than Iodine 103 rows periodic table of electronegativity by pauling scale. Perbromic acid (hbro 4 ). atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. Electronegativity is used to predict whether. → atomic radius decreases → ionization energy increases →. this article contains comparison of key thermal and atomic properties of chlorine. Chlorine More Electronegative Than Iodine.
From slideplayer.com
C and C have the same electronegativity. ppt download Chlorine More Electronegative Than Iodine Electronegativity is used to predict whether. → atomic radius decreases → ionization energy increases →. 119 rows values for electronegativity run from 0 to 4. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. . Chlorine More Electronegative Than Iodine.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Chlorine More Electronegative Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. → atomic radius decreases → ionization energy increases →. Perbromic acid (hbro 4 ). atomic chlorine is much more electronegative than atomic. Chlorine More Electronegative Than Iodine.
From slideplayer.com
Trends in Electronegativity ppt download Chlorine More Electronegative Than Iodine the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. Perbromic acid (hbro 4 ). chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. Electronegativity is used to predict whether. 103 rows periodic table of electronegativity by pauling scale. atomic. Chlorine More Electronegative Than Iodine.
From www.numerade.com
SOLVED Consider the iodine monochloride molecule, ICl . Because Chlorine More Electronegative Than Iodine Perbromic acid (hbro 4 ). the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. → atomic radius decreases → ionization energy increases →. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. 103 rows periodic table of electronegativity. Chlorine More Electronegative Than Iodine.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Chlorine More Electronegative Than Iodine 103 rows periodic table of electronegativity by pauling scale. 119 rows values for electronegativity run from 0 to 4. Perbromic acid (hbro 4 ). → atomic radius decreases → ionization energy increases →. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical elements from the periodic table. Electronegativity is. Chlorine More Electronegative Than Iodine.
From www.slideserve.com
PPT Chapter 17 Reactions of Aromatic Compounds PowerPoint Chlorine More Electronegative Than Iodine chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. Perbromic acid (hbro 4 ). →. Chlorine More Electronegative Than Iodine.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine More Electronegative Than Iodine Perbromic acid (hbro 4 ). the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. chlorine is more reactive than iodine due to its higher electronegativity, resulting from its smaller atomic radius and stronger nuclear. → atomic radius decreases → ionization energy increases →. Electronegativity is used to predict whether. atomic chlorine. Chlorine More Electronegative Than Iodine.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Chlorine More Electronegative Than Iodine the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative than atomic iodine. → atomic radius decreases → ionization energy increases →. this article contains comparison of key thermal and atomic properties of chlorine and iodine, two comparable chemical. Chlorine More Electronegative Than Iodine.
From www.numerade.com
SOLVED What is the product of the addition of ICl to 1 butene? (Hint Chlorine More Electronegative Than Iodine → atomic radius decreases → ionization energy increases →. 119 rows values for electronegativity run from 0 to 4. 103 rows periodic table of electronegativity by pauling scale. the nonmetal chlorine is more electronegative than any other element except fluorine, oxygen, and nitrogen. atomic chlorine is much more electronegative than atomic bromine, which is more electronegative. Chlorine More Electronegative Than Iodine.